Quick guide to Evec
Evec Inc. aims at the development of effective medicine, with less side-effect and less costly to patients, against intractable diseases. We can develop highly effective antibodies, for which no humanization patent cost is charged. It markedly reduces financial burden to patients, and improves the financial management of health insurance.
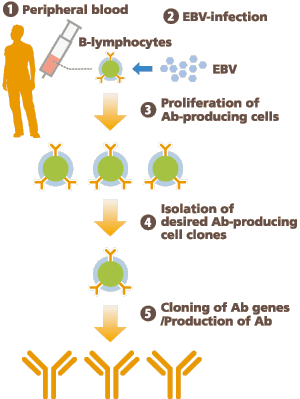
We develop antibodies by using EB virus that induces B-lymphocytes to proliferate.
First, B-lymphocytes are separated from 10~20ml blood, and infected with EB virus.
Then, the cell clones which produce target antibodies are separated from the proliferative B lymphoblastoid cells.
The clones of particular interest are separated by combining the limiting dilution culture and fluorescence-activated cell sorting (FACS). After the separation, the antibody gene is cloned and transferred into CHO cells for antibody production.
The binding activity of our antibodies is ~10-11M, 100 times higher than that of those produced by the mouse immunization method, which is around 10-9M at the highest.
>>For details on how antibodies are produced at Evec, click here

- B-lymphocyte is responsible for antibody production in our body. We are exposed to various antigens after birth, with infection, for example; thus our peripheral B-lymphocyte population can be considered as a library of memory B-lymphocytes potentially producing various antibodies. EB virus has the activity to induce B-lymphocytes to proliferate and to produce antibodies. By using EB virus, proliferation of B-lymphocytes is stably maintained over 6 months.


- Human blood lymphocytes are activated by being naturally exposed to antigens, where a small amount of antigen stimulates them repeatedly, and only those expressing high-affinity antibodies on their surface are accumulated. Evec’s antibodies have much higher binding activity than those currently available in the market.


- Patents on the techniques to develop humanized or fully human antibodies are granted mostly to European and American pharmaceutical companies, and large license fees are charged for their use. While the antibody-developing technique using EB virus has been known over 30 years, its realization has been thought difficult. Evec successfully devised the technique for stable development of antibodies.

>>For further details about the features of Evec technology, click here
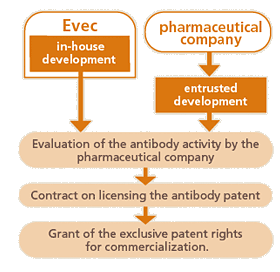

- Evec has developed fully human antibodies by our own technique. We provide our antibodies to companies which commercialize therapeutic monoclonal antibodies.

- Alliance may be formed with the companies aiming to develop new fully human antibodies.
If you are in pharmaceutical business developing new antibody medicine, let us know what kind of human antibodies you need.



