Development of fully human antibodies
- Our mission
- Development of fully human antibodies
- Licensing and joint development of antibodies
- Our strategy
- Evec's Products for Licensing
- Contract Services
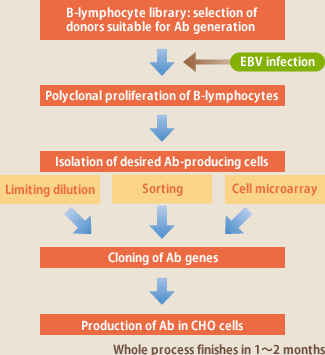
(Fig.1)
Summary
The current methods of antibody development are mainly hybridoma method using mice, and phage display method using E. coli. There are problems with these methods, however, that highly active antibodies are hard to obtain, and that huge amounts of licensing fees are charged by the European or US patent holders.
Evec Inc. established a method to develop antibodies of high affinity and high activity, using Epstein-Barr virus (EBV, which most people are continuously infected with, and has the activity to infinitely proliferate B lymphocytes, i.e., antibody-producing cells). The method is to proliferate B lymphocytes of human blood using EB virus, and isolate those producing aimed antibodies, which have high affinity and high activity acquired through the natural course of immunization in human body.
While the use of EBV for antibody development has been known for over 30 years, culture of lymphocytes and clonal isolation of the cells developing aimed antibodies are difficult, and thus reports of its successful realization are rare. The key features of the Evec's technique are i)accumulated know-how, first of all, how to culture EBV-infected lymphocytes, and others, ii)development of a lymphocyte library consisting of 100 donors' lymphocytes where suitable donors for developing aimed antibodies can be selected in 4 days, and iii)development of sorting method and cell microarray method, to be used additionally to the already available limiting dilution method, for selecting lymphocytes that are producing aimed antibodies (Fig.1). The combination of these has made it possible to develop 20 types of antibody for one target antigen in 6 months. We have so far succeeded in developing highly active antibodies to granulocyte-macrophage colony-stimulating factors(GM-CSF), cytomegalovirus etc.
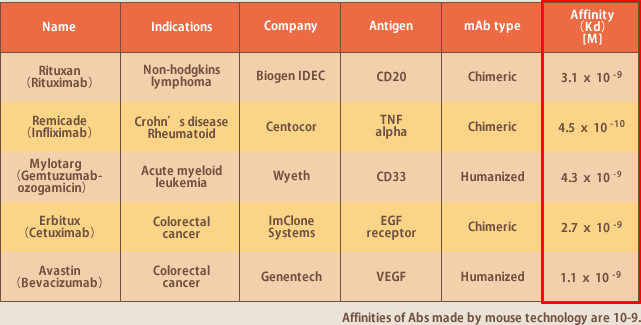
Having little side effects because of their high target specificity, antibodies are expected to be a most effective medicine; among those already in the market, some are achieving annual sales of over 500 billion yen and their market values are estimated to grow further (Fig.2). In fact, most of the techniques of antibody development are patent-granted to the pharmaceutical companies of Europe or the USA, for which use tremendous amounts of licensing fees are charged. Yet the antibodies developed with the best of those techniques do not have satisfactory activities, and the high pharmaceutical cost is also a financial problem of health insurance. Namely, a Japanese own technique to produce antibodies of high activities at low cost is being anticipated.
Evec Inc. has developed a unique technology to produce antibodies using human blood lymphocytes as a sourse of antibody.
(Fig.2)
(Fig.3)
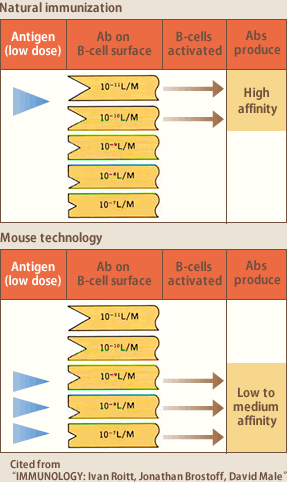
B lymphocytes express antibodies they produce, as membraneous immunoglobulin on their surfaces. An antigen, when entered a human body, binds to the B lymphocytes expressing the antibody to the antigen as membraneous immunoglobulin; consequently the B lymphocytes are activated, proliferate, and differentiate into plasma cells to produce antibodies. For example, if you are infected with influenzavirus, only those B lymphocytes expressing the antibody to the influenzavirus antigen as membranous immunoglobulin are activated to produce a large amount of anti-influenzavirus antibody. If antigen concentration is low, only those B lymphocytes expressing high affinity are activated, reacting to the antigen. If it is high, all the B lymphocytes are activated, regardless of their expression of high or low affinity antibody as a result, the average affinity of the generated antibodies is loe (Fig.3). The former is natural immunization as in flu infection, and the latter (is) short-term immunization as in hybridoma method where mice are artificially immunized with excessive antigen in a short period.
These two types of immunization logically explain why it is difficult to obtain high-affinity antibodies by hybridoma method, and why human blood lymphocytes are an excellent source of high-affinity antibodies. Being repeatedly exposed to antigens of low concentrations, B lymphocytes in human blood develop antibodies of high affinity, and selectively increase their number; namely so-called affinity maturation is constantly occurring.
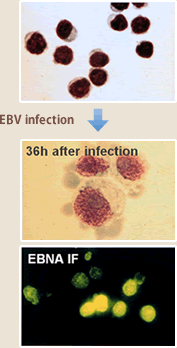
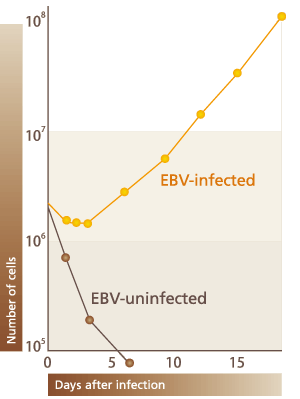
The Epstein-Barr virus (EBV) belongs to the herpes family which includes cytomegalovirus and varicella-zoster virus; most people are infected with EBV before adulthood. Primary infection with EBV is mostly asymptomatic, and adolescent infection can cause infectious mononucleosis, a benign disease of B lymphocyte proliferation. In either case, the virus remains as latent infection in the body in its lifetime, without expressing the virus antigen to be a target of immune system while the body develops its immunity against the virus. Generally the virus is thought to maintain harmless co-habitation with a human body in its lifetime, but in immuno-deficient conditions such as with AIDS or immediately after organ transplantation, EBV-infected B lymphocytes proliferate. Apart from that, links with Burkitt lymphoma, nasopharyngeal carcinoma, T/NK lymphoma, and tec. have been revealed; the virus is one of the human cancer viruses. EBV activation closely related with carcinogenic activation is exemplified by B lymphocyte transforming activation. EBV efficiently infects B lymphocytes by recognizing CD21 as its receptor, which they characteristically express, and induces their proliferation (Fig.4). In optimum, EBV can induce nearly 100% proliferation of B lymphocytes and EBV-infected cells can stably proliferate for more than 6 months.
(Fig.4)
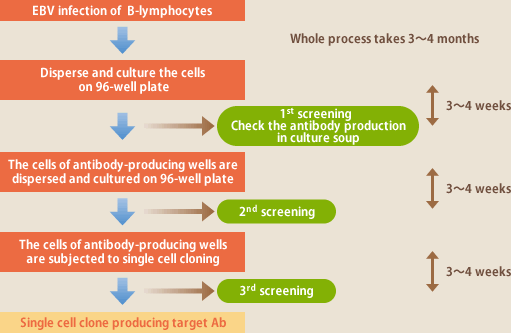
The technique to induce proliferation of human B lymphocytes with the use of EBV and isolate a clone of the lymphocytes producing a particular antibody was reported by Steiniz et al. of Sweden in 1977. Also in Japan, Professor Ono's group of Nihon University reported of their development of various antibodies during the first half of the 1980's. The methods used then were to disseminate EBV-infected B lymphocytes on microplates, culture them and examine whether an aimed antibody was present in the culture soup after 3-4 weeks, and then again disseminate the cells of antibody-positive wells (a mixture of lymphocytes producing different antibodies) on a fresh microplate and incubate them; after repeating these steps, a cell clone which produced an aimed antibody was isolated (Fig.5). The difficulty of this technique is that, if the proliferation speed of aimed- antibody-producing lymphocytes is slower than those of other lymphocytes in the mixture, they will be reduced to be a minor population and disappear through repeated passages. Even if an aimed-antibody-producing clone is isolated, its antibody production or cell proliferation can deteriorate through passages, or most of obtained cells can be IgM-producing, which causes difficulties in handling subsequent antibodies. In those days recombinant techniques were not advanced enough; although there was an idea to use antibodies developed by EBV-infected lymphocytes as a pharmaceutical medicine, it was difficult to completely eliminate contamination with EBV, a cancer virus, which also prevented their pharmaceutical usage.
(Fig.5)
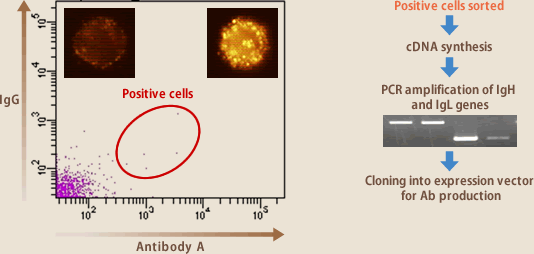
Once a clone of antibody-producing cells is isolated, it is easy today to clone the antibody genes by RT-PCR, and express them in cells such as CHO, and the problems such as proliferation or antibody production of EBV-infected lymphocytes, or contamination with EBV can be completely eliminated now. Namely, the major issue of EBV method is how to isolate aimed-antibody-producing lymphocytes efficiently. Evec has been endeavoring to develop a technique for the issue (Fig.1)
![]()
As mentioned earlier, B lymphocytes express their producing antibodies on the cell surfaces, besides secreting it. It is possible to isolate the lymphocytes producing aimed antibodies, by letting them react to a fluorescence-labeled antigen, using FACS (Fig.6). The same is possible with magnetic beads coated with an antigen. The introduction of the sorting method has made it possible to isolate the lymphocytes producing aimed antibodies, dispensing with the cloning operation by cell culture, and achieving high-speed and efficient antibody production. The method is effective, however, only when a high ratio of lymphocytes are producing aimed antibodies; if the ratio is low, isolation is difficult because aimed- antibody-producing lymphocytes are disturbed by the background of non-specific bindings.
(Fig.6)
![]()
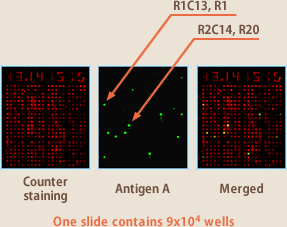
Even more attractive than the sorting method is cell array method. In this method, EBV-infected lymphocytes are dropped on each of 90000 wells of micro-array chip; the wells are covered with slides coated with an antigen and incubated for several hours. Then the slides are fluorescence-stained with anti-human antibody, and array scanner will decide whether the antibodies developed in respective wells react with the antigen. The cells which have developed antibodies to react with the antigen are isolated with a micro-manipulator, and the antibody gene is isolated by RT-PCR (Fig.7, 8). Since the reaction in this method can also be non-specific, it is difficult to isolate particular lymphocytes producing aimed antibodies directly from blood lymphocytes, except in the period from acute stage of infection to immediate after recovery during which the in vivo immunity is in full force. Nonetheless the method has higher accuracy than sorting method, and Evec selectively uses the two for better results of isolating antibody-producing lymphocytes.
(Fig.7)
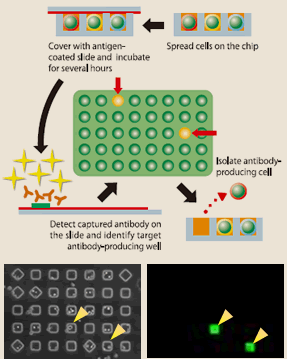
(Fig.8)
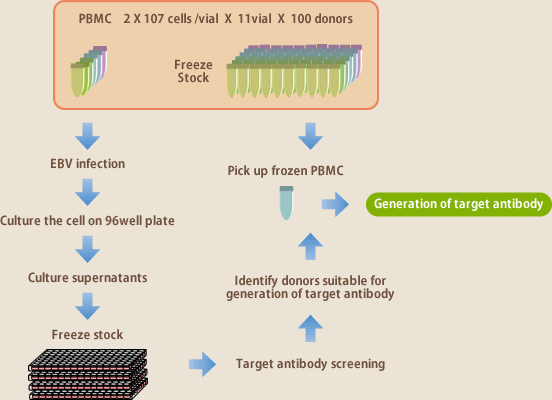
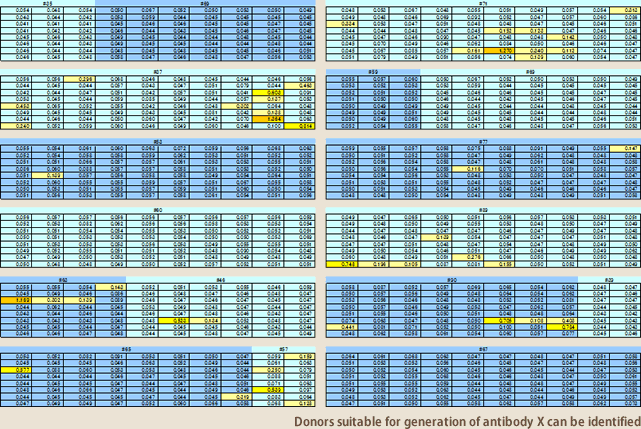
The target of EBV method is memory B lymphocytes. For obtaining an infectious disease antibody, for example, it is the requisite that a donor has experienced the disease. While the measurements of serum antibody titer can tell infection-positive or –negative, the amount of antibody-developing lymphocytes in blood does not completely correlate with serum antibody titer. It is not known actually until the operation of antibody development gets underway whether antibody-producing lymphocytes could be obtained. It is even more difficult to obtain auto-antibodies; depending on a target, it could never be found even if searched in 100 donors. It is impossible with the antibody developing techniques to date to accurately predict whether aimed antibodies could surely be obtained. To make it possible, Evec developed a library consisting of 100 donors' lymphocytes(Fig.9). From each donor, 100~200 ml peripheral blood was collected, and mononuclear cells isolated from the blood were divided to be stored in 11 vials. Ten of them were stored frozen at -152℃, and the cells in the rest 1 vial were infected with EBV, disseminated on 96-well plate; after 3~4 weeks incubation, supernatants were collected respectively from the wells, and stored frozen. In this way, Evec stores the total 100 donors' lymphocytes and culture supernatants. For generating one target antibody, first we examine by ELISA whether aimed antibodies are present in the supernatants of the 100 donors; this operation on the 100 donors is completed in 4 days (Fig.10), and reveals which donors are suitable for generating the antibody. Then the antibody generation is set out using the frozen-stored mononuclear cells of the particular donors where the antibodies have been confirmed to be present. The introduction of this lymphocyte library has speeded up antibody production 10 times faster.
(Fig.9)
(Fig.10)
Evec's antibodies are fully human antibodies, and of high affinity. Therefore there is no need for either humanization from mouse antibodies, or enhancement of activity by phage display method; they have the property ready to be a pharmaceutical medicine. It is also attractive that tremendous license fees for the antibody developing techniques of humanization or activity enhancement are dispensed with. As mentioned, antibodies developed from human lymphocytes have high affinity because blood lymphocytes are undergone affinity maturation in human body. It was proved to be true in our development of a number of antibodies by EBV method. The affinity of the mouse-based antibodies available in the market is in the range of 10-9M; in contrast, a half of our antibodies have 10-11M.
Antibody medicine is expected to flourish further in the future. In the midst of the prospect, human blood lymphocytes are an attractive source of highly affinity antibodies. I am confident that one day the Evec's technique of antibody development will be regarded as a major technique ranked with the mouse method.



